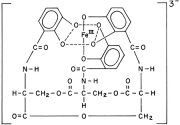
A little over 40 years ago it was discovered that bacteria utilize a class of metal chelators called siderophores to capture iron from their environments. Iron is an important element for the growth and development of most kinds of bacteria. It is used as a critical element in electron transport chains for energy production, the removal of dangerous reactive oxygen species and the functional element in the reactive sites of many important enzymes. The finding was critically important to the field of medicine, as the iron scavenging activities of pathogenic bacteria are most often detrimental to the host. Even though humans and other animals have developed sophisticated systems to sequester iron and make the body inhospitable to these bacteria, likewise they have developed a variety of means of out-competing their hosts for the valuable element. Neisseria gonorrheae and N. meningitidis, for example, have evolved the ability to directly capture iron containing lactoferrin proteins from their human hosts, a testament to our continued co-evolution (1). Still others have relied upon producing their own iron scavenging siderophores to fish out this valuable metal from fluid and protein sources. Escherichia coli , for example, are known for producing enterobactin and have one of the most effective iron scavenging systems known, which at biological pH easily out competes endogenous defenses for iron.
Recently, it has been noted that these compounds are of critical importance to bacterial growth, especially in the ultra-low iron concentrations of bodily tissues. Rather than tighten their belts in the toughest of times, some kinds of pathogenic bacteria turn on
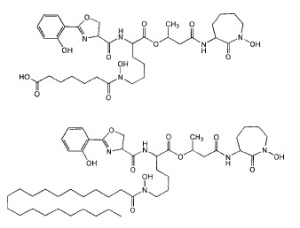
'Like dissolves like': different kinds of Mycobactin are used in different mediums. The long aliphatic chain endows fat solubility to the molecule, allowing it to shuttle through the waxy mycobacterial membrane easily.
these scavenging systems to forcibly acquire the iron needed for growth. Mycobacterium tuberculosis produces several siderophores, called mycobactins, that are medium specific in their activities. The two differ by a water soluble or fat soluble group attached to the siderophore skeleton. Water-soluble mycobactin T is released into aqueous mediums, whereas fat soluble mycobactin T is designed to take pirated iron across the waxy mycobacterial cell membrane (2). When iron is in good supply, however, production of these is unnecessary and is down-regulated to conserve valuable energy and resources. Below the level of production of these siderophores, however, lies a regulated system of control.
Generally siderophore production is controlled by gram negative bacteria’s “Fur” and gram positive’s “DtxR” systems, which have transcription elements that are bound by iron. Thus, when intracellular iron concentrations fall below a threshold (approximately 10^-6 M for many pathogenic bacteria) the iron lynchpin holding back the transcription element is gone, activating the siderophore production system. In 1999, researchers at the University of Queensland showed that Mycobacteria require the production of siderophores for growth in human tissues and macrophages (2). They used genetic engineering techniques to remove the instructions for the enzyme MbtB, which is a critical component of mycobactin synthesis. MtbB effects the final step in mycobactin synthesis, the coupling of a salicylic acid group to the siderophore’s finished structure. After creating an MtbB knockout strain, its ability to produce siderophores was tested by an assay that measured the absorbance of the growth medium. They used a colored dye compound that has a known affinity for iron in solution. If a bacteria were to produce something that has a higher affinity for iron than the dye, this would be measured as a drop in absorbance, as the siderophore would compete with the dye for the iron. They found that after comparing these bacteria against wild type strains that they were unable to produce siderophores and the deficient strains experienced retarded growth both in plate medium and in live macrophages (in vivo vs. in vitro tests).
The understanding of how important these chemicals are for bacterial growth has led to renewed attempts to develop antibiotics against them. In the late 80 and early 90’s the ‘trojan horse’ tactic of using siderophore-antibiotic conjugates and piggybacking them into a cell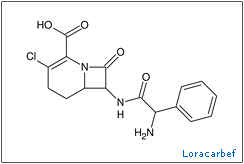 began to bear fruit with successes being discovered in a wide variety human diseases (3)(4)(5). One group of scientists developed conjugates using the carbacephem antibiotic, loracarbef. They found that the mixed catecholate-hydroximate siderophore they used gave the conjugate compound 2,000 times more potency than the parent drug alone (6). Though resistances to this tactic still developed, these are thought to leave the bacteria at a disadvantage, because the mutations hinder the siderophore uptake system rather than attack the antibiotics themselves. This limits access to a vital nutrient and leaves the mutants more susceptible to iron starvation and hindered growth.
began to bear fruit with successes being discovered in a wide variety human diseases (3)(4)(5). One group of scientists developed conjugates using the carbacephem antibiotic, loracarbef. They found that the mixed catecholate-hydroximate siderophore they used gave the conjugate compound 2,000 times more potency than the parent drug alone (6). Though resistances to this tactic still developed, these are thought to leave the bacteria at a disadvantage, because the mutations hinder the siderophore uptake system rather than attack the antibiotics themselves. This limits access to a vital nutrient and leaves the mutants more susceptible to iron starvation and hindered growth.
Though the trojan horse tactic has yielded positive results, there are opportunities for even greater exploitation. Techniques involving small molecules that attack that actual production of siderophores could provide another avenue to beneficial therapy for these diseases. These drugs, much like statins that block the enzyme HMG-coA, would block a critical piece of the siderophore production pathway. In the particular case of mycobactins, the final step in biosynthesis is the attachment of a salicylate group to the mycobactin skeleton. Researchers at Cornell published results in 2005 showing that inhibitors of the enzymes that accomplish this task are potent inhibitors of M. tuberculosis and Yersinia pestis (7). They synthesized a compound, SAL-AMS, that closely resembled a reaction intermediate and measured its effect upon the growth of bacterial cultures in mediums of low iron concentration. They found that it successfully inhibited the enzyme and drastically reduced bacterial growth in the cultures. It was shown to have an IC50(*) of 2.2 ± 0.3 μM for M. tuberculosis and about 51.2 ± 4.7 μM for Y. pestis in an iron limited medium. Though in mediums with high concentration of iron the chemical was ineffective against Y. pestis, but it was found that the chemical might have unknown inhibitory properties against M. tuberculosis, as:
“Salicyl-AMS (tested at up to 8 X IC 50) was not active against Y. pestis in iron-supplemented medium, in which siderophore production is not required for growth. Under these conditions, salicyl-AMS (tested at up to 180 x IC50) did inhibit M. tuberculosis growth, albeit with an 18-fold increase in IC50 (39.9 ± 7.6 μM). This suggests that, in addition to blocking siderophore biosynthesis, salicyl-AMS may also inhibit M. tuberculosis growth by other mechanisms.”
Furthermore, researchers at the university of Minnesota developed similar nucleoside inhibitors of MbtA, one of which ” rivals the first-line antitubercular isoniazid” in activity against the bacteria (8). This tactic follows the same reasoning as SAL-AMP above, as the nucleoside inhibitors attack the same pathway to inhibit siderophore end production. The research was centered around making logical functional modifications to known structures of inhibitors and choosing the most effective ones for further testing.

Comparison of the pathway intermediate, the Cornell inhibitor and one of the Minnesota nucleoside inhibitors.
These findings are coming just in the nick of time it seems, as certain drug resistant strains of M. tuberculosis have become big news recently. Extensively drug resistant tuberculosis is a kind of tuberculosis that is resistant to at least two of the top line drugs used to normally treat it (typically isoniazid and/or rifampicin) and a member of the quinolone antibiotics (ciprofloxacin). Tuberculosis is generally a challenge to treat in the first place, with treatments typically taking up to a year or more to complete. The loss of the first line drugs and reliance upon second line increases the risk of side effects and patient noncompliance to the already long course of therapy. This can further complicate the issue, as it could lead to the obsolescence of the few active drugs used to treat the disease, because resistances to one drug are usually useful against the whole family of drugs.

β-lactamases typically attack the carbonyl in the β-lactam structure, destroying the ring. Nafcillin has a large group that hinders these enzymes from getting too close.
An example of this can be seen in bacteria that produce β-lactamases, as these strains are often cross-resistant to all unprotected β-lactam antibiotics. Some penicillins have been designed with bulky groups attached to the skeleton in an attempt to hinder these enzymes. Nafcillin, with its large 2-ethoxy-1-naphthoyl group, is very effective at blocking these enzymes for the most part. However, even this tactic has its limits as methicillin resistant Staphylococcus aureus (MRSA) and oxacillin resistant Staphylococcus aureus (ORSA), both have developed resistances against these drugs such that, “From 1999 through 2005, the estimated number of S. aureus–related hospitalizations increased 62%, from 294,570 to 477,927 (9),” and “MRSA accounts for an estimated 12% of all nosocomial bacteremias, 28% of surgical wound infections, and 21% of nosocomial skin infections. Infections secondary to MRSA result in excess costs of approximately $4,000 per patient per hospitalization compared with patients infected with methicillin-susceptible S aureus (MSSA)”(10)(11).
Since the discovery of penicillin, Alexander Fleming hypothesized that bacteria would develop resistances to the antibiotics used to treat them. Now, more than ever, the development of new antibiotics must be pursued. Human defenses have always provoked a counter-response from our pathogens, the most successful tactic selected out. Much like the pathogens that infect us, we must ‘evolve’ a newer understanding of bacterial biology, which will offer us a foothold to better, more effective treatments.
——
(1) Genetics and Molecular Biology of Siderophore-Mediated Iron Transport in Bacteria
JORGE H. CROSA
James J. De Voss, Kerry Rutter, Benjamin G. Schroeder, Hua Su, YaQi Zhu, and Clifton E. Barry III
(3) Design, Synthesis, and Study of a Mycobactin−Artemisinin Conjugate That Has Selective and Potent Activity against Tuberculosis and Malaria
Marvin J. Miller, Andrew J. Walz, Helen Zhu, Chunrui Wu,Garrett Moraski, Ute MÖllmann, Esther M. Tristani, Alvin L. Crumbliss, Michael T. Ferdig, Lisa Checkley, Rachel L. Edwards, and Helena I. Boshoff
M. S. DIARRA, M. C. LAVOIE, M. JACQUES, I. DARWISH, E. K. DOLENCE, J. A. DOLENCE, A. GHOSH, M. GHOSH, M. J. MILLER, AND F. MALOUIN
(5) Siderophore-Based Iron Acquisition and Pathogen Control
Miethke M, Marahiel MA.
(6) Iron Transport-Mediated Antibacterial Activity of and Development of Resistance to Hydroxamate and Catechol Siderophore- Carbacephalosporin Conjugates
ALBERT A. MINNICK, JULIA A. McKEE, E. KURT DOLENCE, AND MARVIN J. MILLER
Julian A Ferreras, Jae-Sang Ryu, Federico Di Lello, Derek S Tan & Luis E N Quadri
(8) Antitubercular Nucleosides That Inhibit Siderophore Biosynthesis: SAR of the Glycosyl Domain
SAR of the Glycosyl Domain
Ravindranadh V. Somu, Daniel J. Wilson, Eric M. Bennett, Helena I. Boshoff, Laura Celia, Brian J. Beck, Clifton E. Barry, III, and Courtney C. Aldrich
(10) Baquero F. Gram-positive resistance: Challenge for the development of new antibiotics. J Antimicrob Chemother. 1997;39(suppl A):1–6.
(11) Kopp BJ, Nix DE, Armstrong EP. Clinical and economic analysis of methicillin-susceptible and -resistant Staphylococcus aureus infections. Ann Pharmacother. 2004;38:1377–1382.
(*) The IC 50 is the half maximum inhibitory concentration of antibiotics, a typical measure of effectiveness of the drug.