Muscular dystrophies are a class of degenerative diseases characterized by progressive muscular weakening, myopathy and paralysis. Among these, one of the most famous is Duchenne muscular dystrophy (DMD), which affects about 1 in every 3000 males born in the United States. This class of diseases faces the very limits of medical therapy, as there are currently no clinical treatments available to halt the progression of symptoms. With only supportive care available, patients are left to progressively worsen, typically ending with loss of physical mobility, the ability to breathe independently and usually fatal heart related complications. The disease maligns quality and length of life, such that most only live into their twenties and are confined to a wheelchair around their teens. Only in very rare circumstances have any lived into their 30’s or 40’s.
It wasn’t until fairly recently that an accurate understanding of the pathology involved became clearer. With DMD, mutations from reading frame shifts in the gene producing the dystrophin protein were found to be the root of the problem. Dystrophin is a critical protein involved in muscle structural support and intracellular signaling with the extra-cellular matrix. For any kind of contraction, a muscle must have an anchor and this protein helps play this part by linking the strand to a support structure called the DAP complex, which in turn links to the extracellular matrix. Like a car without shock absorbers, muscle cells are more susceptible to physical membrane damage and slowly whither under the stress. Damaged, leaky membranes are a classic indicator of failed cell viability and seen as the hallmark of dying cells, a fact corroborated by typical diagnostic staining procedures using cell impermeable dyes. Dystrophin is also heavily involved with intracellular signaling from responses outside the cell. Generally, proper signaling is necessary for continued cell viability. Without it cells are usually marked for termination by immune system cells like macrophages, which seek out this kind of aberrant signaling. DMD affected muscles are often infiltrated by these kinds of cells, which further increase the damage by initiation of an inflammatory response.
One of the hallmarks of this disease can be seen in slides of affected muscle. Healthy cells are ensheathed by a thin membrane called the endomysium, but in diseased tissue the spaces around the muscle cells are filled increasingly with fat or fiber-like cartilaginous tissue. This is called endomysial fibrosis. As macrophages realize something is wrong, they infiltrate the area and initiate an immune response causing inflammation. This further weakens the damaged cells and whittles the number of them down slowly, which accounts for the progressive weakening pathology of the disease. The dead cells are typically replaced by this white tissue matrix.
endomysium, but in diseased tissue the spaces around the muscle cells are filled increasingly with fat or fiber-like cartilaginous tissue. This is called endomysial fibrosis. As macrophages realize something is wrong, they infiltrate the area and initiate an immune response causing inflammation. This further weakens the damaged cells and whittles the number of them down slowly, which accounts for the progressive weakening pathology of the disease. The dead cells are typically replaced by this white tissue matrix.
Many hypothesized early on that gene therapy held the greatest promise to cure this disease. After all, in order to truly cure a disease of genes, you must fix those broken genes. Though pharmaceutical treatments reducing immune system response to the disease may improve conditions temporarily, they most likely won’t offer the long-term increase in survivability sought after for so long. As such, many new methods and techniques have been proposed as models for new gene therapy based strategies. Many of these have proven successful in mouse models and a few have even had some success in small clinical trials. These cover a wide range of areas and strategies, such as using viral vectors for gene transplantation, up-regulation of suitable endogenous genes and forced expression of tailored mini genes. Each of these has found success as a possible route towards a therapy, but each with its own drawbacks and limitations. The master stroke, it seems, is still some time off, but even closer and within our child-like grasp at the cookie jar.
- Viral vectors and transgene infection
A now famous example comes from researchers at the University of London. They worked with viral vectors to introduce a dystrophin replacement gene into muscular dystrophic mice. Viral vectors are complicated, because they can provoke a response from the immune system, can possibly cause disease and have a limited storage capacity for any potential gene transplant. They found a balance with the virus called Adeno-associated virus (AAV). These viruses are able to infect many kinds of cells, can be produced without their endogenous viral genes, have never been shown to cause a human disease and also require a helper virus to replicate themselves after infection. This combination of characteristics limits the dangers associated with infection to a degree and makes them good candidates for use in human gene therapies.
One of the main problems they faced was the small storage capacity the virus could carry. The dystrophin gene is one of the largest known (2.4 megabases), so they had to make some kind of compromise. They worked around this by manufacturing a mini-gene that was much smaller than the actual gene that coded for the dystrophin protein. This was made possible by knowledge of the genomes of those affected by the disease. A close cousin of Duchenne muscular dystrophy (DMD) is called Becker muscular dystrophy (BMD) and is characterized by a lesser severity of symptoms. Surprisingly, the gene mutations in BMD, though sometimes ‘affecting ~ 50% of the gene itself’, affect much less critical locations than mutations in DMD. Mapping these locations allowed them to whittle out less critical pieces of the DMD gene. They developed a mini-gene that would produce a lesser functional, but still useful dystrophin protein, which was still able to fit within the size constraints of the viral carrier.
Once the mini-gene was completed they paired it with a transcription promoter from the cytomegalovirus to force gene transcription and production of the protein once the virus infected a cell. They injected the virus into mice expressing a DMD phenotype and found that the mini-gene was successfully expressed in “greater than 50% of the muscle fibers 20 weeks after infection”(S). It was found that it relieved aspects of DMD pathology, particularly rebuilding the DAP complex and improved muscle structure. This culminated with mice test groups showing characteristics of a lesser severe form of the disease. Furthermore, experiments showed that even with a low 20-30% level of gene expression, there was a substantial reduction in DMD pathology overall with the use of this mini gene.
2. Increasing transcription of gene with artificial constructs
As it turns out the dystrophin protein is not the only one utilized to link the muscle to the extracellular network. Before birth, another similar protein called utrophin is used preferentially for the same purpose, but is replaced almost entirely by dystrophin after birth. The protein exhibits 80% similarity with dystrophin and remains expressed during the course of the disease. Researchers at the Istituto di Neurobiologia e Medicina Moleculare in Rome surmised that if preferential expression of utrophin was re-established it might provide a route to a cure or a reduction of symptoms to make it manageable. Using an artificial transcriptional element called “Jazz”, they were able to restore muscle integrity and prevent the development of DMD in mice test groups.
It has long been known that the sudden expression of new, ‘foreign’ proteins runs the risk of causing an immune response as immune cells target these new proteins as antigens. So just giving a person an extra supply of dystrophin won’t work as a treatment for the disease. It was found that when a cell lacks proper functioning dystrophin, it up regulates utrophin to compensate, but the level is insufficient to prevent disease progression. Since this protein is already expressed increased production could reduce complications caused by immune system interference. Many previous studies have confirmed that: “Increased expression of utrophin restores plasma membrane integrity and rescues dystrophin-deficient muscle in mdx mice.”(S)
A ‘zinc finger’ is a recognition element that can interact with specific sections of DNA. In this study, an artificial zinc finger was manufactured to correspond to a section of the promoter in the utrophin gene in both the mice and human genes. Upon testing, they found that it was able to successfully bind to its specific DNA target sequence and increase production of utrophin expression to 1.8 times that of controls. This increase in production also translated to a therapeutic benefit in mice test groups, showing increases in muscle size, fiber regeneration and lower serum levels of creatine kinase, a chemical identifier of muscle necrosis.
Possible benefit was further characterized by in vitro testing of muscles using electro stimulation. Weak muscles, deficient of contractile force are a hallmark of DMD, yet those treated with the ZF ATF “Jazz” showed the opposite in excised diaphragm and extensor digitorum longus muscles. These were able to perform longer and more sustained contractions, than diseased control groups. Membrane integrity was also tested by staining with procion orange dye. This fluorescent dye is usually only taken up into a cell with a leaky membrane, so it is often used to assess membrane integrity. Sustained contractions to a muscle with a DMD phenotype would cause membrane damage and usually exhibit a greater dyed area than that of a healthy cell under the same conditions. Muscles tested and stained under these conditions showed positive results for Jazz treated test sets, with greater dyed areas observed in the DMD cells.
3. Antisense Oligionucleotides:
Antisense oligionucleotides are a class of nucleic acids that have also been tapped as a possible route to a DMD therapy. In 2008, researchers at Oxford published a study testing the hypothesis that these could induce at least partial dystrophin protein expression by pushing the reading frame over in mutated muscle cells. The idea in this technique is to use a technique called ‘exon skipping’ to push the ‘out of reading frame’ portions of the protein back into the reading frame and produce a partially functional, “becker-like” dystrophin protein. This technique was shown to have benefit in not only mice, but also in humans in a proof of concept test in 2007.
Up until now, universal changes in expression from AOs were difficult to attain, with high percentage expression limited to skeletal muscle groups, but only limited expression in critical heart and diaphragm muscles. This was a huge limitation to its possible use as a therapy, as “cardiomyopathy is a significant cause of morbidity and death in DMD patients” (S). Without a corresponding effect upon heart muscle, any increase in overall muscle dystrophin expression would only exacerbate any heart conditions a DMD patient (or mouse) might have. However, this new test was different and achieved much more favorable results in mice. The question is: what did they do different?
It was surmised that the previous tests of AOs had limited entry into exclusive, protected environments like the heart. They needed a tool that would allow access to these areas without destroying the gains made in earlier tests and this was achieved by conjugating the AOs to an arginine rich peptide scaffold. Arginine is a positively charged amino acid and its use in the peptide yields an overall positive charge. These kind of chemicals are thought to use special cell-mediated uptake systems in common with glycosaminoglycans, thus like a Trojan horse they facilitate the entry of whatever cargo they might bring.
Three weeks after injection, “between 25 and 100% of normal dystrophin levels had been restored in body-wide skeletal muscles” and “even in the diaphragm almost 25% of normal dystrophin protein was restored.” Restoration was also seen in the heart, but not quite as high as that of skeletal muscle: “levels between 10 and 20% of that found in normal mouse heart were typically seen in all western analysis in all treated animals.” These results also corresponded with a function increase in muscle contractility and lower serum creatine kinase levels, both indicators of improved muscle ability and reduced muscle damage.
To build a tower on the sea.
Though these seem like giant steps towards a cure for one of the great diseases of man, there will undoubtedly be more questions than answers. These are exciting times.
Every one of these people worked really hard. Please read these sources first hand (at least these):
1. “Adeno-associated virus vector gene transfer and sarcolemmal expression of a 144 kDa micro-dystrophin effectively restores the dystrophin-associated protein complex and inhibts myofibre degeneration in nude/mdx mice.“ Stewart A. Fabb, Dominic J. Wells, Patricia Serpente, george Dickson. Human Molecular Genetics, 2002, vol. 11, No. 7, pgs 733-741.
2. “Expression of human full-length and minidystrophin in transgenic mdx mice: implications for gene therapy of Duchenne muscular dystrophy.” Wells DJ, Wells KE, Asante EA, Turner G, Sunada Y, Campbell KP, Walsh FS, Dickson G. Hum Mol Genet. 1995 Aug;4(8):1245-50.
3. “The artificial gene Jazz, a transcriptional regulator of utrophin, corrects the dystrophic pathology in mdx mice.” Maria Grazia Di Certo, Nicoletta Corbi, Georgios Strimpakos, Annalisa Onori, Siro Luvisetto, Cinzia Severini, Angelo Guglielmotti, Enrico Maria Batassa, Cinzia Pisani, Aristide Floridi, Barbara Benassi, Maurizio Fanciulli, Armando Magrelli, Elisabetta Mattei, and Claudio Passananti. Hum. Mol. Genet. (2010) 19 (5): 752-760.
4. “Cell-penetrating peptide-conjugated antisense oligionucleotides restore systemic muscle and cardiac dystrophin expression and function.” HaiFang Yin, Hong M. Moulton, Yiqi Seow, Corinne Boyd, Jordan Boutilier, Patrick Iverson and Matthew J.A. Wood. Hum. Mol. Genet. (2008) 17 (24): 3909-3918.
5. “Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy.” Hong Li, Ashwani Mittal. Denys Y. Makonchuk, Shephali Bhatnagar and Ashok Kumar. Hum. Mol. Genet. (2009) 18 (14): 2584-2598.





 SAR of the Glycosyl Domain
SAR of the Glycosyl Domain


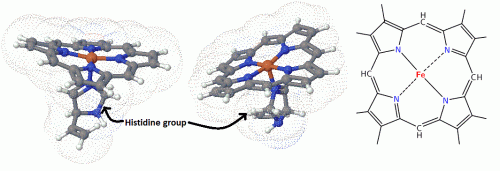
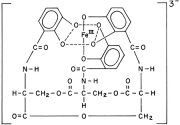
 Jorgensen believed that the ligands in compounds like (CoCl3 * 4 NH3) were arranged in chains, that is, bonded to each other in some fashion. The main point being that the atoms would follow known valence rules at the time, especially
Jorgensen believed that the ligands in compounds like (CoCl3 * 4 NH3) were arranged in chains, that is, bonded to each other in some fashion. The main point being that the atoms would follow known valence rules at the time, especially 



