One of the most recent advances in cancer therapy has been the development of new drugs inhibiting a class of enzymes called  tyrosine kinases. Normally, these enzymes are involved with the transduction of signals involving the growth and proliferation of a cell. In cancer cells, however, their activity is greatly increased such that they gain increased ability to proliferate and spread general nastiness, in contrast to the ordered and regulated growth of normal cells. In 2005, one of the first drugs of the class, imatinib, was lauded by time magazine and the New York Times as a great leap forward in cancer treatment and “converted a fatal cancer into a manageable chronic condition.” [NYtimes] With few treatment options besides a bone marrow transplant, chronic myelogenous leukemia was one of the more fatal kinds of cancer. However, with these new therapies researchers have shown that “after 60 months of Gleevec (imatinib’s brand name) therapy, 98% of patients had shown a complete hematologic response. Also at 60 months, the estimated overall survival rate for patients was 89%, with a relapse rate of only about 17%.” [1]
tyrosine kinases. Normally, these enzymes are involved with the transduction of signals involving the growth and proliferation of a cell. In cancer cells, however, their activity is greatly increased such that they gain increased ability to proliferate and spread general nastiness, in contrast to the ordered and regulated growth of normal cells. In 2005, one of the first drugs of the class, imatinib, was lauded by time magazine and the New York Times as a great leap forward in cancer treatment and “converted a fatal cancer into a manageable chronic condition.” [NYtimes] With few treatment options besides a bone marrow transplant, chronic myelogenous leukemia was one of the more fatal kinds of cancer. However, with these new therapies researchers have shown that “after 60 months of Gleevec (imatinib’s brand name) therapy, 98% of patients had shown a complete hematologic response. Also at 60 months, the estimated overall survival rate for patients was 89%, with a relapse rate of only about 17%.” [1]
However, a word of caution must be spread. These drugs are not the often sought after ‘cure for cancer,’ as patients still run the risk of relapse and mutation fueled resistance to the therapies, as well as the controversy considering the incredible cost of treatment (imatinib costs $32-98K per year for CML). That said, their development is still a milestone in our understanding of cancer pathology and its treatment. Much of the class was developed out of what is called ‘rational design’ methodology, that is to say by using deductive means based upon knowledge of the shape and function of the principal components of the systems. This is opposed to drug screening strategies, used during much of the earlier history of drug discovery, of finding a lucky diamond in the rough and modifying it to fit certain ‘structural activity relationships’. Direct knowledge of the target areas and their effects has allowed medicinal chemists to design new approaches from the ground up to develop more effective treatments for disease. Perhaps, as the knowledge base becomes deeper and more comprehensive, we might be able to attack the real root of the issue in this terrible disease, but for the time being we must be patient with the fruits that our limited knowledge provides.
What exactly is a tyrosine kinases and what does it do?
Tyrosine kinases are enzymes that act as chauffeurs of a chemical signal, that when passed along to other mediaries can effect some kind of action in a cell. These actions are many and varied. In relation to cancer, these are typically signals to allow growth, cell division or the creation of signals to induce the production of new blood vessels. When activated, tyrosine kinases usually pass the signal down to relay proteins that activate the downstream machinery for these actions, in effect acting as an ‘on/off switch’ for that particular activity.
Cells have many different kinds of RTKs and we must distinguish between two different general classes when considering these drugs. Receptor tyrosine kinases are membrane bound receptors for a long list of growth factors, that in response to the extracellular binding of the factor, the receptor becomes activated and allows the association with other signaling proteins by self-phosphorylating tyrosine residues in the trans-membrane and C-terminal regions of the protein’s structure. These create docking spaces for downstream signaling proteins, like Ras, STAT and PI3K, allowing larger signaling complexes to form and the information to be passed on. Much like a Dremel tool with a selection of interchangeable parts, these kinds of signaling proteins can modulate the signal sent and create a whole network of interactions within the system. For example, when a Ras protein is activated by the receptor interaction can induce a MAP kinase cascade that culminates with protein transcription in the nucleus by unlocking a large set of transcription factors. PI3K is involved with cell survival and insulin induced metabolic control. Likewise, STAT proteins can be activated in this fashion and move into the nucleus where they become active transcription factors. There are also non-Receptor tyrosine kinases, which are usually located in the cytoplasm or nucleus instead of the cell membrane. Two of the most common are c-src and abl, which are involved in a similar array of growth regulating, cell maintaining activities. The abl tyrosine kinase, for example, is usually located in the nucleus and is involved in the repair of DNA damage and regulation of apoptosis and transcription. Src is involved heavily in the regulation of cell division and differentiation in many cells.
Where cancer comes in is the fact that some mutated or viral versions of these enzymes are constitutively active, that is to say, stuck in the on position. This activity is in part responsible for the explosive growth seen in some tumors. Some cancer inducing retroviruses, like the Rous sarcoma virus or murine Abelson leukemia virus, are known to produce these kinds of kinases as part of their payload. In fact, the first tyrosine kinase to be discovered was called ‘v-src kinase’, denoting where it had come from. The WHO has estimated that around 17% of all human cancers might be caused by these kinds of rouge viral kinases. [2] Furthermore, in 1972 Janet Rowley noticed that patients with a rare and deadly form of cancer called chronic myelogenous leukemia had a chromosomal abnormality later named the Philadelphia chromosome. This was caused by the transplantation of two genes ‘abelson tyrosine kinase’ and ‘break point cluster’ creating a chimeric gene of the two called Bcr-Abl. The fusion protein created by this gene is another ‘always on’ version of the enzyme and was where these new therapies got their start. Though these enzymes are ubiquitous and normal cells do have an Abl kinase, Bcr-Abl is unique enough that drugs can be targeted towards it and other cell kinase redundancies can cover for its loss of function. This affords a more specific therapy against these cancer cells than other earlier chemotherapy based strategies.
Where da Drugs At?
Since the tyrosine kinases require ATP for activation, quite a few of these compounds were developed from the idea to ‘poison the well’  with a similar looking, yet difficult to remove compound at the ATP binding site. The early research drug semaxanib was developed for this purpose and led to the development of the ‘non-receptor tyrosine kinase inhibitor’ sunitinib, and the first ever dog specific anti-cancer drug: toceranib (Yeah, you read that right, “priorities”). Each of these has similarity with the structure of ATP built right into the compound, which is close enough to enable them to bind to the enzyme’s active site.
with a similar looking, yet difficult to remove compound at the ATP binding site. The early research drug semaxanib was developed for this purpose and led to the development of the ‘non-receptor tyrosine kinase inhibitor’ sunitinib, and the first ever dog specific anti-cancer drug: toceranib (Yeah, you read that right, “priorities”). Each of these has similarity with the structure of ATP built right into the compound, which is close enough to enable them to bind to the enzyme’s active site.
Though semaxanib is a natural product, the majority of these compounds are purely synthetic and were developed through direct knowledge of the enzyme structure and its interactions. Knowledge of the binding space has allowed designers to make judicious choices in functional groups to elicit specific bonding to the enzyme. One interesting point of note is the use of highly electronegative groups, like fluorine atoms, to anchor and bind to amino acids in the enzyme’s superstructure. The reasoning is twofold in that this enables better targeting of the compound to the receptor space via interaction with polar amino acid groups and makes them more difficult to remove from the enzyme in natural conditions. The drugs regorafenib and sorafenib are good examples of this, which include a trifluoro-methane group (-CF3) that is intended to anchor these compounds in the right place.
Considering current human therapies, first line therapies inhibiting cancers involving mutations to epidermal growth factor receptors, like erlotinib and gefitinib, have long been newsworthy. Therapies attacking non-receptor tyrosine kinases, like abl kinase and it’s CML fusion cousin, are exemplified by Imatinib, dasatinib and nilotinib. These are targeted to block excessive receptor signaling by damaged kinases of these receptors. . Resistances, however, are apparent even with these new therapies, as a single point mutation in the gene encoding the enzyme removes their effectiveness and reinstates bcr-abl activity. The T315I point mutation changes a single amino acid (threonine to isoleucine) in the enzyme and is the most prevalent resistance found among resurgent CML, occurring in perhaps “6 out of every 9 cases of imatinib resistance.” [3] This mutation confers resistance to not only imatinib, but also others of the class, due to its critical location guarding the binding pocket of the enzyme. Dasatinib and nilotinib represent the second generation therapies over imatinib, which, in the case of nilotinib has 30 times the binding efficacy in imatinib resistant cancer cells at the bcr-abl binding site. Furthermore, in the case of non-small cell lung carcinoma, a small percentage of people have a similar mutation creating a fusion protein called EML4-ALK, which is similarly specifically targeted by the drug crizotinib. [4]
Vascular endothelial growth factor (VEGF) is involved with the new production of blood vessels around a tumor that provide if with the additional blood flow and nutrition needed for increased growth. This “angiogenesis” has long been implicated as a critical event in the development of deadly tumors and prognosis can even be intuitively estimated by the amount of VEGF in a sample and thus vascularization of a given tumor. As luck would have it, VEGF receptors have in common a tyrosine kinase that we have recently learned to inhibit with the drugs pazopanib, axitanib and vandetanib. Pazopanib has been approved for use against renal cell carcinoma and has shown moderately positive results in phase II studies involving its use against soft tissue sarcomas and ovarian cancer. [5] Axitanib has been also tested against metastatic renal cell carcinoma with positive results as: “axitinib increased median progression-free survival time by 43% among the overall patient population, from 4.7 months to 6.7 months (maximum 8.6 months). Importantly, benefits of the drug were evident in patients who had previously been treated with cytokines or sunitinib.” [6] Vandetanib has likewise been tested against a kind of thyroid cancer with the “estimated progression-free survival for the patients treated with vandetanib was 30.5 months.” [7]
Final thoughts
Though our knowledge of cancer pathology has clearly increased to point to effectively manipulate specific areas of weakness in the cancer cell machinery, developing resistances and the sheer cost continue to remain a challenges for widespread effective therapy.
[NYTimes] Interesting NY Times interview with Druker.
[1] “After 5 years of follow-up, continuous treatment of chronic-phase CML with imatinib as initial therapy was found to induce durable responses in a high proportion of patients.”
[2]“The estimated total of infection-attributable cancer in the year 2002 is 1.9 million cases, or 17.8% of the global cancer burden. The principal agents are the bacterium Helicobacter pylori (5.5% of all cancer), the human papilloma viruses (5.2%), the hepatitis B and C viruses (4.9%), Epstein-Barr virus (1%), human immunodeficiency virus (HIV) together with the human herpes virus 8 (0.9%). Relatively less important causes of cancer are the schistosomes (0.1%), human T-cell lymphotropic virus type I (0.03%) and the liver flukes (0.02%).”
[3] CML point mutations study.
[4] “The primary endpoint of both trials was objective response rate (ORR) as assessed by the investigator. In Study A, the ORR was 50 percent (95 percent CI: 42 percent, 59 percent) with a median response duration of 42 weeks. In Study B, the ORR was 61 percent (95 percent CI: 52 percent, 70 percent) with a median response duration of 48 weeks. Complete responses were observed in 1 percent of patients. No differences in ORR by performance status, number of prior chemotherapeutic regimens, or percentage of cells found to have the ALK gene rearrangement were noted.”
[5] Admittedly, this is a shitty source without links to useful information, but hey, at least it has impressive looking numbers that look like it has information to back up its claims. I’m very sorry for including this in here, as laziness has gotten the better of me.
[6] “The Phase III Axis 1032 study included 723 patients, who were randomized to receive either axitinib or sorafenib twice daily. 54% of patients had previously been treated using sunitinib-containing regimens, and 35% had received prior cytokine-based therapy. The results showed that compared with sorafenib therapy, axitinib increased median progression-free survival time by 43% among the overall patient population, from 4.7 months to 6.7 months (maximum 8.6 months). Importantly, benefits of the drug were evident in patients who had previously been treated with cytokines or sunitinib, Pfizer notes.
In previously cytokine-treated patients, axitinib therapy led to a median progression-free survival of 12.1 months, compared with 6.5 months for the sorafenib group. Among previously sunitinib-treated patients, progression-free survival was 4.8 months for those moved on to axitinib therapy, compared with 3.4 months for those given sorafenib. Secondary trial endpoints showed that the independently assessed objective response rate was 19.4% among the overall axitinib patient cohort, compared with 9.4% for the sorafenib cohort. “
[7] (http://www.cancer.gov/clinicaltrials/results/summary/2011/vandetanib1211)






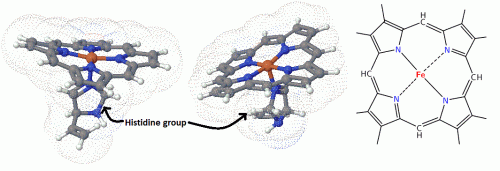



 SAR of the Glycosyl Domain
SAR of the Glycosyl Domain




 One more thing, I almost forgot the sauce. People usually dip them in some kind of sauce, variations of which are almost endless. You can
One more thing, I almost forgot the sauce. People usually dip them in some kind of sauce, variations of which are almost endless. You can 

